Breaking the chain of cross contamination
By Dr Kayleigh Cox-Nowak, Technical Support Manager, Schülke & Mayr UK Ltd.
Effective infection prevention strategies are essential in the dental setting, where there is a high risk of cross-contamination [DePaola, 2019; Upendran 2020]. The use of water air sprays, high-speed handpieces, ultrasonic instruments and air polishers are all aerosol generating procedures (AGP). If a patient has an infection, the aerosols and droplets produced have the potential to contaminate surfaces and become reservoirs of potential infection [Harrell, 2004; Rautemaa, 2006].
Hand hygiene and surface disinfection are key to preventing the transmission of pathogens and there is evidence to strongly link the two. Making contact with a contaminated surface and not performing hand hygiene adequately can facilitate the transmission of pathogens. Likewise, poor hand hygiene can lead to surface contamination.
Contaminated surfaces are an established route of transmission for both bacteria and viruses; potentially resulting in onward contamination of hands or equipment [Otter, 2016].
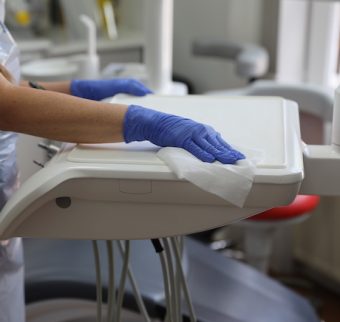
Breaking the chain of contamination – surfaces
Surfaces can become contaminated by hands, objects, and the settling of pathogen containing aerosols or contaminated fluids [Becker, 2019]. Viral persistence on different types of inanimate surfaces is well documented and includes dental chairs, hand rests, tables, drawers, plastic and wooden surfaces, switches, light handles, latex, cotton, tissues, gloves, disposable gowns, steel objects, and dental instruments [Otter, 2016; Peng 2020]. Pathogen transfer can be interrupted by the appropriate cleaning and disinfection of surfaces; with disinfectant wipes having a key role to play [Sattar, 2013].
Disinfectant wipes are frequently used in healthcare settings, like dental surgeries, as they are quick and convenient to use, but there is considerable variation in efficacy between different wipes. Given the links between environmental contamination and pathogen transfer, it is important to select the most appropriate wipes.
To ensure the efficacy of a disinfectant wipe against viruses like SARS-CoV-2 there is a recognised microbiological test for virucidal activity, according to the European Norm (EN) standard EN14476. A positive result means that the solution within the wipes/tissue is efficacious against enveloped viruses. Choosing a wipe with ‘virucidal efficacy against enveloped viruses’ combined with mention of EN14476 is a useful indicator for the selection of the disinfection wipe.
Passing the EN14476 test means that the disinfectant solution can kill viruses like SARS-CoV-2, but what influences the practical application of the wipe is the required contact time. This is the time required for the solution to be in contact with pathogens in order to eliminate or inactivate them.
A disinfectant wipe could be EN14476 certified but may require a contact time of 5 minutes to be effective, compared to another requiring only 30 seconds. A long contact time is more likely to damage a surface over a period of time, than a shorter one. A shorter contact time will save time, help simplify the cleaning/disinfection process and is likely to make compliance easier to adhere to. The EN16615 test is the highest level of testing for antimicrobial wipes under the recognition of the European Standards committee. The test examines the efficacy of the wipe as a whole i.e. the wipe plus the disinfectant component.
When selecting a wipe for the clinical setting, it cannot be assumed that all wipes will have this ‘gold standard’ EN16615 certification. A wipe like mikrozid has both EN14476 and EN16615 certification. However, many wipes will have EN14476 certification without EN16615, which should raise questions about whether these wipes are fit for purpose in a dental practice.
Breaking the chain of contamination – hands
It is recommended that hand hygiene products are manufactured for clinical use, as these products are generally unscented, have fewer allergenic components, and are formulated to be used repeatedly throughout the day. Alcohol-based hand rubs (ABHR) kill microorganisms more effectively and more quickly than hand washing with soap and water. They are also less damaging to skin, resulting in less dryness and irritation. They are also more accessible and may enhance compliance. However, they do not physically remove debris from hands and should not be used if hands are visibly soiled [Fluent, 2013].
Hand hygiene with (ABHR) is widely accepted as one of the most effective, simple and low-cost procedures to help prevent cross-transmission of pathogens. By denaturing proteins, alcohol inactivates enveloped viruses such as coronaviruses. When exposed to an ethanol-based disinfectant, the SARS-CoV-2 virus on human skin is completely inactivated within 15 seconds [Hirose, 2020].
The alcohol component of the hand rub is the active ingredient that eliminates microorganisms. Besides its rapid killing action, the fast drying time is also an advantage when it comes to hand sanitising. 60 -70% alcohol (commonly ethanol/isopropanol) is the concentration demonstrated to be effective against enveloped viruses, such as those causing COVID-19 [CDC, 2020].
The actual formulation of an ABHR is critical. The antimicrobial agents within the product need to work in conjunction with added components like moisturiser, without compromising each other. An ABHR for use in a dental practice should conform to stringent testing, for example European Norms (EN): EN 1500, EN 12791 and EN 14476.
European Norm (EN) 1500 is utilised in Europe for testing of ABHRs to reduce the level of microbes for normal hand hygiene practices whereas EN 12791 applies to surgical hand hygiene. Both EN tests require healthy test volunteers and the hand rub being tested is challenged against a reference active agent. This is a rigorously defined procedure to examine the efficacy of a particular hand disinfectant. EN 14476 evaluates virucidal activity and is a specific in vitro test of formulations against viruses including enveloped viruses, like SARS-CoV-2.
There are significant differences in efficacy between products that have been certified in accordance with the applicable European standards, compared to non-certified products. A study comparing a certified ABHR with a non-certified one showed that even after participants had been trained to EN 1500 standards in hand hygiene, the bacterial burden was only reduced by 6-fold from baseline using the non-certified product compared to close to 50-fold from baseline with the certified one [Babulek, 2014].
A useful starting point therefore for selecting an ABHR is to check if it conforms to EN standards. For example, desderman® pure gel meets EN1500 for hygienic hand disinfection in 30 seconds and EN12791 for surgical hand disinfection in 90 seconds as well as EN14476 for virucidal efficacy.
When selecting an ABHR for use, it is essential that it is formulated with additional moisturisers and re-fattening agents to help protect the hands. This is also likely to improve compliance as the ABHR is not drying out the hands. In a clinical environment staff use a hand sanitiser nine times an hour, on average [Albright, 2018].
Since the COVID-19 pandemic began, clinical grade ABHRs have sometimes been in short supply due to the surge in demand. This has led to a number of ‘new’ ABHR manufacturers who often have little or no experience of supplying medical grade products. Considerable caution therefore needs to be exercised in selecting an ABHR for clinical use. A substandard ABHR could have undesired consequences, such as sub-optimal antimicrobial efficacy and skin incompatibility for staff and patients.
An ABHR has recently been withdrawn from use in Ireland when some batches were found to contain methanol rather than ethanol. A health warning was issued that ‘prolonged use of the product may cause skin problems, eye and respiratory irritation and headaches.’ The hand sanitiser has since been withdrawn from Ireland’s biocidal product register [BBC, October 2020].
Breaking the chain of hands and environmental cross contamination, using effective EN certified products is a key strategy in protecting both staff and patients from infection.
References
Albright J, Use patterns and frequency of hand hygiene in healthcare facilities: Analysis of electronic surveillance data, American Journal of Infection Control 2018, 46, 1104-9
Babulek R et al, Hand hygiene–evaluation of three disinfectant hand sanitizers in a community setting, PLoS One. 2014 Nov 7;9(11)
BBC https://www.bbc.co.uk/news/uk-northern-ireland-54660217 accessed 291020
Becker B, Henningsen L, Paulmann D, et al. Evaluation of the virucidal efficacy of disinfectant wipes with a test method simulating practical conditions. Antimicrob. Resist. Infect. Control. 2019; 8:121 doi.org/10.1186/s13756-019-0569-4
2019,8, 1–8.
Boyce JM. Environmental contamination makes an important contribution to hospital infection. J Hosp Infect. 2007;65 Suppl 2:50-54. doi:10.1016/S0195-6701(07)60015-2
CDC Statement for Healthcare Personnel on Hand Hygiene during the Response to the International Emergence of COVID-19, 14th March 2020
https://www.cdc.gov/coronavirus/2019-ncov/infection-control/hcp-hand-sanitizer.html
accessed 29th October 2020
DePaola, L. G., Grant, L. E. (2019). Summary of infection control in the dental office: A global prospective. Infection Control in the Dental Office: A Global Perspective, 213–216. https://doi.org/10.1007/978-3-030-30085-2_15
Fluent MT. Hand hygiene in the dental setting: reducing the risk of infection. Compend Contin Educ Dent. 2013;34(8):624-627
Harrel, S. K., Molinari, J. (2004). Aerosols and splatter in dentistry: A brief review of the literature and infection control implications. Journal of the American Dental Association, 135(4), 429–437. https://doi.org/10.14219/jada.archive.2004.0207
Hirose R, Ikegaya H, Naito Y, et al. Survival of SARS-CoV-2 and influenza virus on the human skin: Importance of hand hygiene in COVID-19 [published online ahead of print, 2020 Oct 3]. Clin Infect Dis. 2020;ciaa1517. doi:10.1093/cid/ciaa1517
Otter JA, Donskey C, Yezli S, Douthwaite S, Goldenberg SD, Weber DJ. Transmission of SARS and MERS coronaviruses and influenza virus in healthcare settings: the possible role of dry surface contamination. J Hosp Infect. 2016;92(3):235-250. doi:10.1016/j.jhin.2015.08.027
Peng, X., Xu, X., Li, Y., Cheng, L., Zhou, X., Ren, B. (2020). Transmission routes of 2019-nCoV and controls in dental practice. International Journal of Oral Science, 12(1), 9. https://doi.org/10.1038/s41368-020-0075-9
Rautemaa, R., Nordberg, A., Wuolijoki-Saaristo, K., Meurman, J. H. (2006). Bacterial aerosols in dental practice—A potential hospital infection problem? Journal of Hospital Infection, 64(1), 76–81. https://doi.org/10.1016/j.jhin.2006.04.011
Sattar SA, Maillard JY. The crucial role of wiping in decontamination of high-touch environmental surfaces: review of current status and directions for the future. Am J Infect Control. 2013;41:S97–104.
Upendran, A., Geiger, Z. (2020, February 17). Dental infection control. https://www.ncbi.nlm.nih.gov/books/NBK470356/